Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Suzuki, Satoru; Kawamura, Katsuyuki*
JNC TN8400 2001-005, 41 Pages, 2001/04
A correlation between molecular structure and a vibrational spectrum of interlayer water in Na-smectite was investigated by means of Molecular Dymamics (MDs) simulations. Detailed comparison of simulation results with IR spectroscopic observations for the water-smectite system indicated good agreement. Internal vibrational spectra of water were obtained by the Fourier transformation of velocty auto-correlation function of hydrogen atom. A stretching vibrational spectrum of interlayer water consisted of a broad band with a peak top around 3400cm and a sharp peak around 3650 to 3700cm
and a sharp peak around 3650 to 3700cm . The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O
. The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O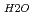 -O
-O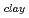
 3.0
3.0  -O
-O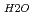 = ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
= ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
Suzuki, Satoru; ; *
JNC TN8400 2000-020, 25 Pages, 2000/04
Nature of porewater in bentonite plays important roles on the mass transport in the compacted bentonite used as a physical and chemical buffer material of the multi-barrier system in the high level radioactive waste manegement Higher activation energies of diffusion in the compacted bentonite than those in the aqueous solution is due probably to change in molecular structure of water in the porewater. The Raman spectroscopy was applied to studying the structure of porewater in bentonite at room temperature. Bentonite (Kunipia F, 98-99wt% of Na-smectite) was mixed with ion-exchanged water by water content of 75, 80, 90, 95 and 98wt% of water or with 0.5M NaCl aqueous solution by 75 and 80wt% of NaCl solution. Intensity maxima of the spectra of ion exchanged water, NaCl solution and their porewater were observed near 3200 to 3250, 3400, 3630cm . These bands can be attributed to water molecules forming stronger hydrogen bond in this manner. Ratio of intensity, 3250cm
. These bands can be attributed to water molecules forming stronger hydrogen bond in this manner. Ratio of intensity, 3250cm /3400cm
/3400cm , increased from 0.97 to 1.1 with a decrease in water content of 100wt% (water) to 75wt%. On the other hand, intensity ratio of 3400cm
, increased from 0.97 to 1.1 with a decrease in water content of 100wt% (water) to 75wt%. On the other hand, intensity ratio of 3400cm /3250cm
/3250cm of NaCl aqueous solution, 80wt%and 75wt% were 0.92, 1.2 and 1.3, respectively. Since the Raman scattering near 3250cm
of NaCl aqueous solution, 80wt%and 75wt% were 0.92, 1.2 and 1.3, respectively. Since the Raman scattering near 3250cm was attributed to water molecule forming the strongest hydrogen bonding in the three bands, those changes in intensity ratio suggests an increase in number of water molecule forming strong hydrogen bond in porewater of the bentonite. The constrained porewater possibly results in the high activation energy of diffusion in the compacted bentonite.
was attributed to water molecule forming the strongest hydrogen bonding in the three bands, those changes in intensity ratio suggests an increase in number of water molecule forming strong hydrogen bond in porewater of the bentonite. The constrained porewater possibly results in the high activation energy of diffusion in the compacted bentonite.
Sato, Tsutomu
Sumekutaito Kenkyukai Kaihou, 7(2), p.39 - 41, 1997/00
no abstracts in English
Sato, Tsutomu
Kobutsugaku Zasshi, 25(3), p.99 - 110, 1996/07
no abstracts in English
Sato, Tsutomu
SMECTITE, 6(1), p.45 - 48, 1996/00
no abstracts in English
Sato, Tsutomu
SMECTITE, 6(2), p.33 - 36, 1996/00
no abstracts in English